Project Description
Background
A pharmaceutical client’s manufacturing plant required high purity water for manufacturing of their range of various pharmaceutical products. The end user group, founded in 2005, provides end-to-end solutions from research to product launch in a variety of segments like Pharmaceuticals, Nutraceuticals, Ayurveda and sports nutrition. The group has multiple leading-edge advanced formulation manufacturing facilities throughout India.
The water source for the plant is brackish well water. The process scheme chosen to treat this water to generate high purity water consisted of pretreatment followed by two pass RO and electrodeionization.
QUA Solution
FEDI Model: FEDI-2-20Rx
FEDI Stacks: 2
Flow: 3.8 m˜ /hr
Application: USP grade water for Pharmaceutical Manufacturing
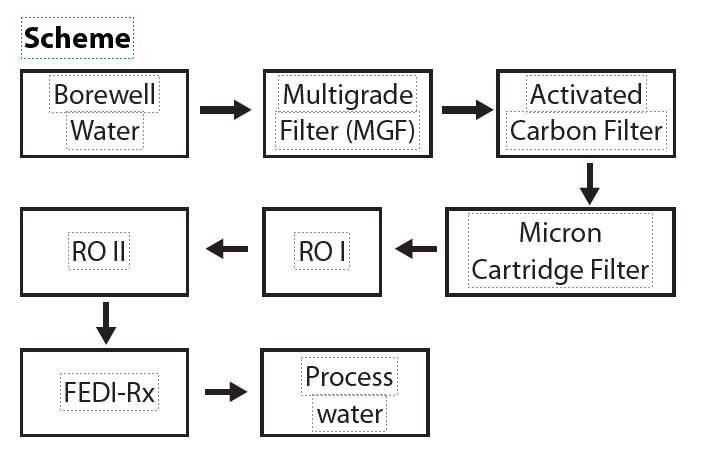
After a detailed technical evaluation, the client determined that QUA’s FEDI®-Rx would be the best fit for their requirement. FEDI-Rx series stacks are typically used in USP grade purified water/WFI (water for injection) systems for pharmaceutical applications. These stacks can be hot water sani-tized at 85°C water temperature. The wetted components of FEDI-Rx stacks confirm to US FDA requirements. The stacks are CE certified and comes with triclover end connections. FEDI-Rx stacks can produce water quality up to 18 megaOhms. FEDI has many successfully operating installations in pharmaceutical applications. Many of these have been in operation continuously for many years, delivering high grade purified water.
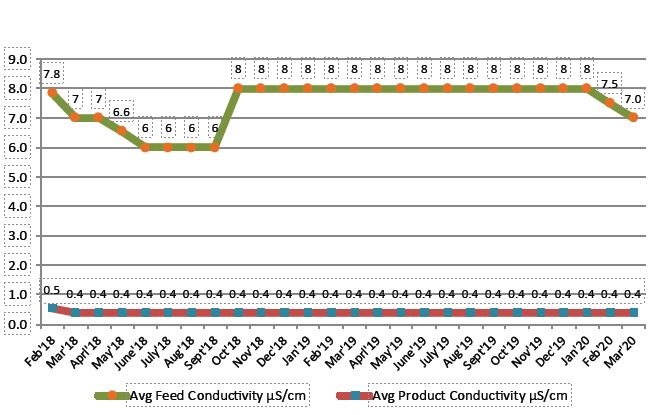
QUA supplied 2 of its FEDI-2-20Rx stacks, which were commissioned in November 2015, and have been delivering consistent high purity water of conductivity less than 0.5 microS/cm since.
The client has been very satisfied with the performance of the FEDI system and the product water quality has met their norms. They have subsquently installed FEDI for similar applications for pharmaceutical manufacturing.
Results
The following graph represents FEDI-Rx’s performance over the years.
FEDI Product Conductivity Consistently Less than 0.5 microS/cm