Project Description
Background
A leading global pharmaceutical company had an existing water treatment system that they were using to produce USP grade water; consisting of a softener, 2 pass reverse osmosis system and elec-trodeionization (EDI) as the final polisher to achieve water conductivity as per standards.
The client’s existing electrodeionization system, with another manufacturer’s EDI, was not able to deliver consistent product water quality. The feed water quality was ˛fluctuating, and their existing EDI stack failed due to extensive scaling. The client’s production was adversely affected. Service also became a key issue as the customer was not satisfied with the existing EDI manufacturer’s support and service. They started exploring other EDI options, and expected their new EDI supplier to understand the problem and work closely to resolve quality concerns as soon as possible.
The client required an EDI system,
which could deliver consistent product water quality on a continuous basis irrespective of variation in feed water conditions
that could be an easy retrofit to replace the existing system.
with a low lead time for delivery, since production was impacted.
QUA Solution
FEDI Model: FEDI-2-20Rx
FEDI Stacks: 1
Flow: 2 m˜ /hr
Application: USP grade water for Pharmaceutical application
After a detailed technical evaluation, the OEM and the client selected QUA’s FEDI-2-20Rx as the easy retrofit solution. FEDI-Rx series stacks are typically used in USP grade puriÿed water/WFI systems. These stacks can be hot water sanitized at 85°C water temperature. The wetted components of FEDI-Rx stacks confirm to US FDA requirements. The stacks are CE certified and comes with triclover end connections. FEDI-Rx stacks can produce water quality upto 18 megaOhms.
Besides the superior technology, the factor that convinced the client that FEDI Rx was the right solution for their requirement was the pre-sales support provided by QUA’s engineers and technical support team. QUA’s technical engineers visited the site multiple times to evaluate the client’s entire system and suggested modifications to be carried out in the system. This support was important to the client to ensure they selected a trusted partner who could help them solve their high purity water production issues. Once FEDI-Rx was selected, QUA delivered the stack quickly, since delivery time was critical to ensure uninterrupted production at the site. QUA’s engineers supported the end-user during installation, and were present at site throughout commissioning; the client’s operations team was given comprehensive training to ensure confidence in daily operation. QUA has subsequently been closely monitoring the site along with the client’s operations team, to ensure trouble free operation of the FEDI system.
Results
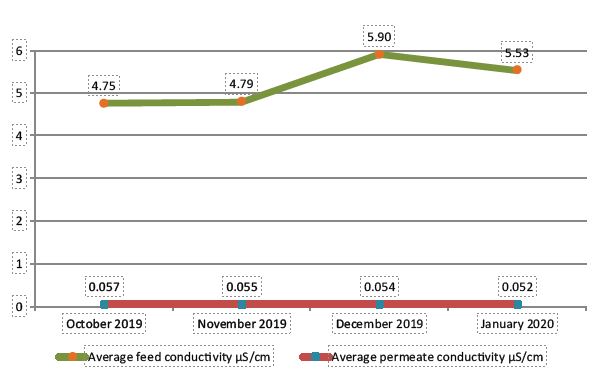
The FEDI Rx system was commissioned in October 2019, and has been delivering consistent high purity water of conductivity less than 0.1 microS/cm since then.
The client has been so impressed with FEDI’s performance and QUA’s service support, that they have also retrofitted their ultrafiltration membrane system with QUA’s Q-SEP® 6008 UF membranes.